Current Therapeutic Strategies for Metastatic Triple-Negative Breast Cancer: From Pharmacists’ Perspective
Abstract
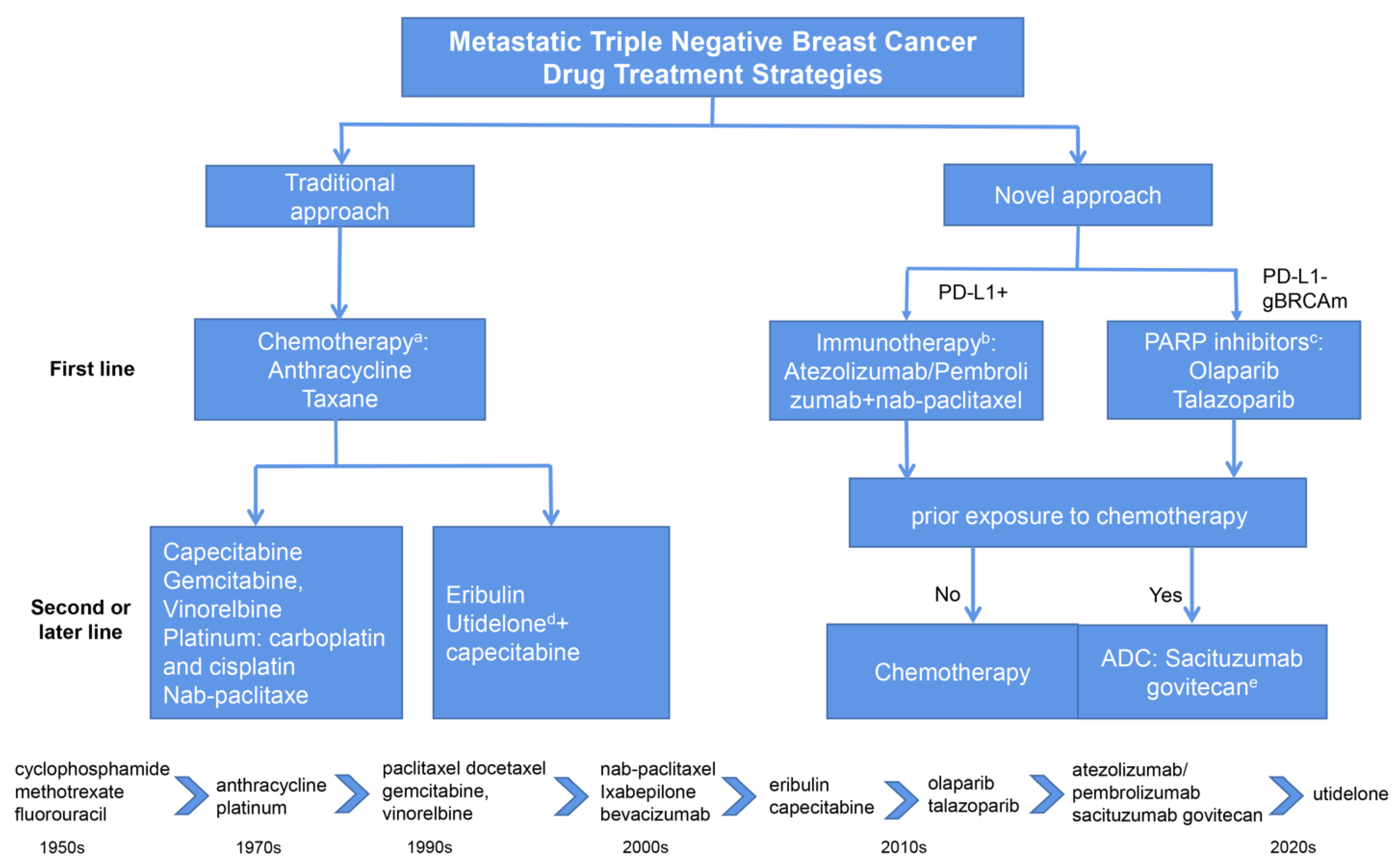
:1. Introduction
2. Traditional Chemotherapy Regimens
3. Novel Chemotherapy Agents
3.1. Utidelone
| Phase | Intervention | Line of Therapy | Patients | N | Efficacy | Safety (AE ≥ Grade 3) |
|---|---|---|---|---|---|---|
| Phase 2 trial [26] | Utidelone + capecitabine vs. utidelone | Second or later-line | mBC | 33 | ORR: 42.4% vs. 28.57%; PFS: 7.9 vs. 5.4 months | Peripheral neuropathy: 45.5% vs. 8.6%; hand-foot syndrome: 15.2% vs. 0%; hematologic toxicity: 6.1% vs. 7.1%; myalgia and arthralgia: 15.2% vs. 1.4 |
| Phase 3 trial [23] | Utidelone + capecitabine vs. capecitabine | Second or later-line | mBC refractory to anthracycline and taxane | 405 | PFS: 8.44 vs. 4.27 months | Peripheral sensory neuropathy: 22% vs. < 1%; palmar-plantar erythrodysaesthesia: 7% vs. 8% |
| Phase 1/2 trial [31] | Eribulin + olaparib | Second or later-line | Advanced or metastatic TNBC | 48 | ORR: 37.5%; PFS: 4.2 months; OS: 14.5 months | Leucopenia: 87.5%; anemia: 41.7; neutropenia: 87.5%; febrile neutropenia: 33.3%; diarrhea: 4.2% |
| Phase 3 trial [32] | Eribulin vs. TPC | Third or later-line | mBC | 762 | ORR:12% vs. 5%; OS: 13.1 vs. 10.6 months; PFS: 3.7 vs. 2.2 months | Neutropenia: 21% vs. 14%; leucopenia: 12% vs. 5%; fatigue: 8% vs. 10%; peripheral neuropathy: 8% vs. 2%; dyspnea: 4% vs. 2% |
| Phase 3 trial [33] | Eribulin vs. vinorelbine | Third or later-line | mBC | 530 | ORR: 30.7% vs. 16.9%; OS: 13.4 vs. 12.5 months; PFS: 2.7 vs. 1.4 months | Total: 88.3% vs. 90.2%; anemia: 2.3% vs. 18.3%; febrile neutropenia: 2.7% vs. 1.2% |
| Phase 3 trial [34] | Eribulin vs. capecitabine | First-, second-, or third-line | mBC | 1102 | ORR: 11.0% vs. 11.5%; OS: 15.9 vs. 14.5 months; PFS: 4.1 vs. 4.2 months | Neutropenia: 24.6% vs. 4.2%; leukopenia:13.4% vs. 1.8%; Anemia: 2.0% vs. 0.9%; peripheral neuropathy: 6.4% vs. 0.9% |
| Phase 2 trial [35] | Eribulin + gemcitabine | First- or second-line | mTNBC | 83 | ORR: 37.3%; OS: 14.5 months; PFS: 5.1 months | Aminotransferase elevation: 25%; Neutropenia: 23.8% |
| Phase 2 trial [36] | Eribulin + bevacizumab | Second-line | HER2-negative mBC | 58 | ORR: 24.6%; OS: 14.8 months; PFS: 6.2 months | Hypertension: 7%; neutropenia: 7%; febrile neutropenia: 7% |
| Phase 2 trial [37] | Eribulin | First- or second-line | mBC | 32 | ORR: 43.8%; PFS: 8.3% | Neutropenia: 40.6%; peripheral neuropathy: 12.5%; fatigue: 12.5%; thrombopenia: 6.3% |
| Phase 2 trial [38] | Eribulin | Second-line | mBC | 53 | ORR: 20.8%; CBR: 26.4%; | Neutropenia: 35.9%; leukopenia:17% |
| Phase 2 trial [39] | Camrelizumab + apatinib + eribulin | NR | Advanced TNBC | 46 | ORR: 37%; PFS: 8.1 months; DCR: 87% | Elevated AST/ALT: 17.4%; neutropenia: 30.4%; leukopenia: 13.0%; thrombocytopenia: 19.6% |
| Phase 2 trial [40] | Eribulin + gemcitabine vs. Paclitaxel + gemcitabine | First-line | HER2-negative mBC | 118 | ORR: 48.9% vs. 44.1%; the 6-months PFS rate: 72% vs. 73% | Neutropenia: 57.6% vs. 67.8%; neurotoxicity: 13.6% vs. 45.8% |
| Phase 1b/2 trial [41] | Eribulin + pembrolizumab | First-line and later-line | mTNBC | 167 | PD-L1+ vs. PD-L1−: (1) first-line: ORR: 34.5% vs. 16.1%; (2) second or later-line: ORR: 24.4% vs. 18.2% | Neutropenia: 26%; immune-related AE: 12% |
3.2. Eribulin Mesylate
4. Novel Targeted Therapeutic Agents
4.1. ADCs
4.2. PARP Inhibitor
5. Immune Checkpoint Inhibitors (ICIs)
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [Green Version]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.D.; Liu, Y.R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440. [Google Scholar] [CrossRef] [Green Version]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Moy, B.; Rumble, R.B.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.R.; Hortobagyi, G.N.; Yee, D.; Smith, I.E.; Chavez-MacGregor, M.; et al. Chemotherapy and Targeted Therapy for Patients with Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That is Either Endocrine-Pretreated or Hormone Receptor-Negative: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3938–3958. [Google Scholar] [CrossRef]
- Nandini, D.; Jennifer, A.; Pradip, D. Therapeutic Strategies for Metastatic Triple-Negative Breast Cancers: From Negative to Positive. Pharmaceuticals 2021, 14, 455. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Park, S.; Kwon, Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol. Ther. 2019, 199, 30–57. [Google Scholar] [CrossRef]
- Lu, P.; Santa-Maria, C.A.; Ballinger, T.J.; Sheng, J.Y. Landmark trials in the medical oncology management of metastatic breast cancer. Semin. Oncol. 2021, 48, 246–258. [Google Scholar] [CrossRef]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Caparica, R.; Lambertini, M.; de Azambuja, E. How I treat metastatic triple-negative breast cancer. ESMO Open 2019, 4, e000504. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hu, Y.; Tang, C.; Guan, X.; Zhang, W. Platinum-based systematic therapy in triple-negative breast cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188678. [Google Scholar] [CrossRef]
- Yu, K.D.; Ye, F.G.; He, M.; Fan, L.; Ma, D.; Mo, M.; Wu, J.; Liu, G.Y.; Di, G.H.; Zeng, X.H.; et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women with Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1390–1396. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Ponde, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, B.H.; Yuan, P.; Ma, F.; Wang, J.Y.; Ding, X.Y.; Zhang, P.; Li, Q.; Cai, R.G. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann. Oncol. 2013, 24, 1219–1225. [Google Scholar] [CrossRef]
- Hu, X.C.; Zhang, J.; Xu, B.H.; Cai, L.; Ragaz, J.; Wang, Z.H.; Wang, B.Y.; Teng, Y.E.; Tong, Z.S.; Pan, Y.Y.; et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 436–446. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.H.; Hu, X.; Wang, B.; Wang, L.; Yang, W.; Liu, Y.; Liu, G.Y.; Di, G.H.; Hu, Z.I.; et al. Cisplatin and gemcitabine as the first line therapy in metastatic triple negative. Int. J. Cancer 2015, 136, 204–211. [Google Scholar] [CrossRef]
- Priyanka, S.; Eve, R.; William, E.B.; Julie, G.; Shannon, L.H.P.; Carey, K.A.; Lori, J.G.; Ursa, A.B.G.; Thu-Tam, H.; Christopher, S.S.; et al. Results of a phase II randomized trial of cisplatin +/− veliparib in metastatic triple-negative breast cancer (TNBC) and/or germline BRCA-associated breast cancer (SWOG S1416). J. Clin. Oncol. 2020, 38, 1001. [Google Scholar]
- Fumoleau, P.; Coudert, B.; Isambert, N.; Ferrant, E. Novel tubulin-targeting agents: Anticancer activity and pharmacologic profile of epothilones and related analogues. Ann. Oncol. 2007, 9, v9–v15. [Google Scholar] [CrossRef]
- Thomas, E.S.; Gomez, H.L.; Li, R.K.; Chung, H.C.; Fein, L.E.; Chan, V.F.; Jassem, J.; Pivot, X.B.; Klimovsky, J.V.; de Mendoza, F.H.; et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J. Clin. Oncol. 2007, 25, 5210–5217. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, L. Breast cancer: Utidelone: Burden relief in pretreated women. Nat. Rev. Clin. Oncol. 2017, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, T.; Zhang, Q.; Yuan, Z.; Jiang, Z.; Wang, X.J.; Cui, S.; Teng, Y.; Hu, X.C.; Yang, J.; et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: A multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 371–383. [Google Scholar] [CrossRef]
- Xu, B.; Sun, T.; Zhang, Q.; Zhang, P.; Yuan, Z.; Jiang, Z.; Wang, X.; Cui, S.; Teng, Y.; Hu, X.C.; et al. Efficacy of utidelone plus capecitabine versus capecitabine for heavily pretreated, anthracycline- and taxane-refractory metastatic breast cancer: Final analysis of overall survival in a phase III randomised controlled trial. Ann. Oncol. 2021, 32, 218–228. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, M.Y.; Qiu, R.G.; Tang, L.; Dou, G.; Xu, B. Phase I clinical and pharmacokinetic study of UTD1, a genetically engineered epothilone analog in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 971–978. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Z.; Tian, F.; Wang, Y.; Yang, J.; Li, W.; Di, L.; Liu, W.; Tang, L.; Qiu, R.; et al. Phase II trial of utidelone as monotherapy or in combination with capecitabine in heavily pretreated metastatic breast cancer patients. J. Hematol. Oncol. 2016, 9, 016–0297. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, Y.; Jiang, C.; Cao, H.; Jiang, J.; Xu, B.; Sun, T. Ganglioside Monosialic Acid Alleviates Peripheral Neuropathy Induced by Utidelone Plus Capecitabine in Metastatic Breast Cancer From a Phase III Clinical Trial. Front. Oncol. 2020, 10, 524223. [Google Scholar] [CrossRef]
- Bao, T.; Seidman, A.D.; Piulson, L.; Vertosick, E.; Chen, X.; Vickers, A.J.; Blinder, V.S.; Zhi, W.I.; Li, Q.; Vahdat, L.T.; et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur. J. Cancer 2018, 101, 12–19. [Google Scholar] [CrossRef]
- Müller, J.; Weiler, M.; Schneeweiss, A.; Haag, G.M.; Steindorf, K.; Wick, W.; Wiskemann, J. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: A randomised-controlled trial. Br. J. Cancer 2021, 125, 955–965. [Google Scholar] [CrossRef]
- Liao, M.; Jiang, Q.; Hu, H.; Han, J.; She, L.; Yao, L.; Ding, D.; Huang, J. Cost-effectiveness analysis of utidelone plus capecitabine for metastatic breast cancer in China. J. Med. Econ. 2019, 22, 584–592. [Google Scholar] [CrossRef]
- Yonemori, K.; Shimomura, A.; Yasojima, H.; Masuda, N.; Aogi, K.; Takahashi, M.; Naito, Y.; Shimizu, S.; Nakamura, R.; Hashimoto, J.; et al. A phase I/II trial of olaparib tablet in combination with eribulin in Japanese patients with advanced or metastatic triple-negative breast cancer previously treated with anthracyclines and taxanes. Eur. J. Cancer 2019, 109, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T.; et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Yuan, P.; Hu, X.; Sun, T.; Li, W.; Zhang, Q.; Cui, S.; Cheng, Y.; Ouyang, Q.; Wang, X.; Chen, Z.; et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: A randomised clinical trial. Eur. J. Cancer 2019, 112, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, P.A.; Awada, A.; Twelves, C.; Yelle, L.; Perez, E.A.; Velikova, G.; Olivo, M.S.; He, Y.; Dutcus, C.E.; Cortes, J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2015, 33, 594–601. [Google Scholar] [CrossRef]
- Pellegrino, B.; Cavanna, L.; Boggiani, D.; Zamagni, C.; Frassoldati, A.; Schirone, A.; Caldara, A.; Rocca, A.; Gori, S.; Piacentini, F.; et al. Phase II study of eribulin in combination with gemcitabine for the treatment of patients with locally advanced or metastatic triple negative breast cancer (ERIGE trial). Clinical and pharmacogenetic results on behalf of the Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC). ESMO Open 2021, 6, 100019. [Google Scholar]
- De Angelis, C.; Bruzzese, D.; Bernardo, A.; Baldini, E.; Leo, L.; Fabi, A.; Gamucci, T.; De Placido, P.; Poggio, F.; Russo, S.; et al. Eribulin in combination with bevacizumab as second-line treatment for HER2-negative metastatic breast cancer progressing after first-line therapy with paclitaxel and bevacizumab: A multicenter, phase II, single arm trial (GIM11-BERGI). ESMO Open 2021, 6, 100054. [Google Scholar] [CrossRef]
- Hayashida, T.; Jinno, H.; Mori, K.; Sato, H.; Matsui, A.; Sakurai, T.; Hattori, H.; Takayama, S.; Wada, M.; Takahashi, M.; et al. Phase II trial of eribulin mesylate as a first- or second-line treatment for locally advanced or metastatic breast cancer: A multicenter, single-arm trial. BMC Cancer 2018, 18, 701. [Google Scholar] [CrossRef] [Green Version]
- Ortega, V.; Anton, A.; Garau, I.; Afonso, N.; Calvo, L.; Fernandez, Y.; Martinez-Garcia, M.; Blanco, E.; Zamora, P.; Garcia, M.; et al. Phase II, Multicenter, Single-arm Trial of Eribulin as First-line Therapy for Patients with Aggressive Taxane-pretreated HER2-Negative Metastatic Breast Cancer: The MERIBEL Study. Clin. Breast Cancer 2019, 19, 105–112. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tian, Z.; Lin, Y.; Li, H.; Zhu, Z.; Liu, Q.; Su, S.; Zeng, Y.; Jia, W.; et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat. Commun. 2022, 13, 3011. [Google Scholar] [CrossRef]
- Park, Y.H.; Im, S.A.; Kim, S.B.; Sohn, J.H.; Lee, K.S.; Chae, Y.S.; Lee, K.H.; Kim, J.H.; Im, Y.H.; Kim, J.Y.; et al. Phase II, multicentre, randomised trial of eribulin plus gemcitabine versus paclitaxel plus gemcitabine as first-line chemotherapy in patients with HER2-negative metastatic breast cancer. Eur. J. Cancer 2017, 86, 385–393. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.M.; Kaufman, P.A.; Wilks, S.T.; Andreopoulou, E.; et al. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin. Cancer Res. 2021, 27, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; Correia, J.J. Macromolecular interaction of halichondrin B analogues eribulin (E7389) and ER-076349 with tubulin by analytical ultracentrifugation. Biochemistry 2009, 48, 7927–7938. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Vahdat, L.T. Eribulin mesylate. Clin. Cancer Res. 2011, 17, 6615–6622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivot, X.B.; Marmé, F.; Koenigsberg, R.; Guo, M.; Berrak, E.; Wolfer, A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann. Oncol. Adv. Access 2016, 27, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Mergui-Roelvink, M.; Wanders, J.; Jenner, A.; Edwards, G.; Reyderman, L.; Copalu, W.; Peng, F.; Marchetti, S.; Beijnen, J.H.; et al. Eribulin mesylate pharmacokinetics in patients with solid tumors receiving repeated oral ketoconazole. Investig. New Drugs 2013, 31, 381–389. [Google Scholar] [CrossRef]
- Ro, J.; Cheng, F.T.; Sriuranpong, V.; Villalon, A.; Smruti, B.K.; Tsang, J.; Yap, Y.S.; Asian Working Group for Eribulin Clinical Guide. Patient Management with Eribulin in Metastatic Breast Cancer: A Clinical Practice Guide. J. Breast Cancer 2016, 19, 8–17. [Google Scholar] [CrossRef]
- Devriese, L.A.; Witteveen, P.O.; Marchetti, S.; Mergui-Roelvink, M.; Reyderman, L.; Wanders, J.; Jenner, A.; Edwards, G.; Beijnen, J.H.; Voest, E.E.; et al. Pharmacokinetics of eribulin mesylate in patients with solid tumors and hepatic impairment. Cancer Chemother. Pharmacol. 2012, 70, 823–832. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (accessed on 5 August 2022).
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients with Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer_Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results_ Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negativer. Med. Oncol. 2019, 30, 558–566. [Google Scholar]
- Eikesdal, H.P.; Yndestad, S.; Elzawahry, A.; Llop-Guevara, A.; Gilje, B.; Blix, E.S.; Espelid, H.; Lundgren, S.; Geisler, J.; Vagstad, G.; et al. Olaparib monotherapy as primary treatment in unselected triple negative breast. Ann. Oncol. 2021, 32, 240–249. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Tischkowitz, M.; Mackay, H.; Swenerton, K.; Robidoux, A.; Tonkin, K.; Hirte, H.; Huntsman, D.; Clemons, M.; Gilks, B.; et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011, 12, 852–861. [Google Scholar] [CrossRef]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Telli, M.L.; Rugo, H.S.; Mailliez, A.; Ettl, J.; Grischke, E.M.; Mina, L.A.; Balmaña, J.; Fasching, P.A.; Hurvitz, S.A.; et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO). Clin. Cancer Res. 2019, 25, 2717–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gatalica, Z. Trop-2 protein as a therapeutic target: A focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers. Bosn. J. Basic Med. Sci. 2022, 22, 14–21. [Google Scholar] [CrossRef] [PubMed]
- M-Rabet, M.; Cabaud, O.; Josselin, E.; Finetti, P.; Castellano, R.; Farina, A.; Agavnian-Couquiaud, E.; Saviane, G.; Collette, Y.; Viens, P.; et al. Nectin-4: A new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 2017, 28, 769–776. [Google Scholar] [CrossRef]
- Sharkey, R.M.; McBride, W.J.; Cardillo, T.M.; Govindan, S.V.; Wang, Y.; Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Enhanced Delivery of SN-38 to Human Tumor Xenografts with an Anti-Trop-2-SN-38 Antibody Conjugate (Sacituzumab Govitecan). Clin. Cancer Res. 2015, 21, 5131–5138. [Google Scholar] [CrossRef] [Green Version]
- Wahby, S.; Fashoyin-Aje, L.; Osgood, C.L.; Cheng, J.; Fiero, M.H.; Zhang, L.; Tang, S.; Hamed, S.S.; Song, P.; Charlab, R.; et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin. Cancer Res. 2021, 27, 1850–1854. [Google Scholar] [CrossRef]
- TRODELVY® (Sacituzumab Govitecan-Hziy) for Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761115s005s013lbl.pdf (accessed on 22 April 2020).
- Baek, G.; Jung, L.; Duong, A.; Gralow, J. Case report of sacituzumab govitecan-hziy-induced neutropenia in a patient with metastatic triple-negative breast cancer and a uridine diphosphate glucuronosyltransferase family 1 member A1 poor metabolizer genotype. J. Oncol. Pharm. Pract. 2022, 28, 710–716. [Google Scholar] [CrossRef]
- Lyons, T.G. Targeted Therapies for Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2019, 20, 82. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-olaparib-gbrcam-her2-negative-metastatic-breast-cancer (accessed on 12 January 2018).
- Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer (accessed on 16 October 2018).
- Hurvitz, S.A.; Quek, R.G.W.; Turner, N.C.; Telli, M.L.; Rugo, H.S.; Mailliez, A.; Ettl, J.; Grischke, E.M.; Mina, L.A.; Balmaña, J.; et al. Quality of life with talazoparib after platinum or multiple cytotoxic non-platinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations: Patient-reported outcomes from the ABRAZO phase 2 trial. Eur. J. Cancer 2018, 104, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, L.; Gai, D.; He, S.; Jiang, X.; Zhang, N. Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Front. Pharmacol. 2022, 13, 851246. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Postel-Vinay, S.; Im, S.A.; Park, Y.H.; Delord, J.P.; Italiano, A.; Alexandre, J.; You, B.; Bastian, S.; Krebs, M.G.; et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020, 21, 1155–1164. [Google Scholar] [CrossRef]
- Lee, J.M.; Peer, C.J.; Yu, M.; Amable, L.; Gordon, N.; Annunziata, C.M.; Houston, N.; Goey, A.K.; Sissung, T.M.; Parker, B.; et al. Sequence-Specific Pharmacokinetic and Pharmacodynamic Phase I/Ib Study of Olaparib Tablets and Carboplatin in Women’s Cancer. Clin. Cancer Res. 2017, 23, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Konstantinopoulos, P.A.; Cheng, S.C.; Supko, J.G.; Polak, M.; Wahner-Hendrickson, A.E.; Ivy, S.P.; Bowes, B.; Sawyer, H.; Basada, P.; Hayes, M.; et al. Combined PARP and HSP90 inhibition: Preclinical and Phase 1 evaluation in patients with advanced solid tumours. Br. J. Cancer 2022, 126, 1027–1036. [Google Scholar] [CrossRef]
- Zhao, D.; Long, X.; Wang, J. Metabolism-related pharmacokinetic drug-drug interactions with poly (ADP-ribose) polymerase inhibitors. Oncol. Rep. 2022, 47, 23. [Google Scholar] [CrossRef]
- LYNPARZA® (Olaparib) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s001lbl.pdf (accessed on 12 January 2018).
- Morice, P.M.; Leary, A.; Dolladille, C.; Chrétien, B.; Poulain, L.; González-Martín, A.; Moore, K.; O’Reilly, E.M.; Ray-Coquard, I.; Alexandre, J. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: A safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021, 8, e122–e134. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef]
- Zou, Y.; Zou, X.; Zheng, S.; Tang, H.; Zhang, L.; Liu, P.; Xie, X. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940928. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [Green Version]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Winer, E.P.; Lipatov, O.; Im, S.A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; Andre, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.S.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Lee, J.S.; Yost, S.E.; Frankel, P.H.; Ruel, C.; Egelston, C.A.; Guo, W.; Gillece, J.D.; Folkerts, M.; Reining, L.; et al. A Phase II Clinical Trial of Pembrolizumab and Enobosarm in Patients with Androgen Receptor-Positive Metastatic Triple-Negative Breast Cancer. Oncologist 2021, 26, 24. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer with 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Narayan, P.; Wahby, S.; Gao, J.J.; Amiri-Kordestani, L.; Ibrahim, A.; Bloomquist, E.; Tang, S.; Xu, Y.; Liu, J.; Fu, W.; et al. FDA Approval Summary: Atezolizumab Plus Paclitaxel Protein-bound for the Treatment of Patients with Advanced or Metastatic TNBC Whose Tumors Express PD-L1. Clin. Cancer Res. 2020, 26, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple (accessed on 13 November 2020).
- Schmid, P.; Salgado, R.; Park, Y.H.; Munoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Qu, J.; Jiang, M.; Wang, L.; Zhao, D.; Qin, K.; Wang, Y.; Tao, J.; Zhang, X. Mechanism and potential predictive biomarkers of immune checkpoint inhibitors in NSCLC. Biomed. Pharmacother. 2020, 127, 109996. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; de Azambuja, E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials—How to explain different results? ESMO Open 2020, 5, e001112. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wong, D.; Vogel, A.S.; Sack, J.S.; Rahma, O.E.; Hodi, F.S.; Zucker, S.D.; Grover, S. Effect of corticosteroid dosing on outcomes in high-grade immune checkpoint inhibitor hepatitis. Hepatology 2022, 75, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Chang, B.; Pham, A.; Chan, A. Impact of pharmacist-managed immune checkpoint inhibitor toxicities. J. Oncol. Pharm. Pract. 2021, 27, 596–600. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]


| Phase | Intervention | Line of Therapy | Patients | N | Efficacy | Safety (AE ≥ Grade 3) |
|---|---|---|---|---|---|---|
| Phase 3 trial [53] | Sacituzumab govitecan vs. single-agent chemotherapy | Second- or later-line | mTNBC | 468 | ORR: 35% vs. 5%; OS: 12.1 vs. 6.7 months; PFS: 5.6 vs. 1.7 months | Neutropenia: 51% vs. 33%; leukopenia: 10% vs. 5%; anemia: 8% vs. 5%; febrile neutropenia: 6% vs. 2%; diarrhea: 10% vs. <1% |
| Phase 1/2 trial [52] | Sacituzumab govitecan | Third- or later-line | mTNBC | 108 | ORR: 33.3%; OS: 13.0 months; PFS: 5.5 months | Neutropenia: 39%; leukopenia: 16%; diarrhea: 13%; vomiting and hypophosphatemia: 10%; |
| Phase 1 trial [55] | Trastuzumab deruxtecan | NR | advanced/metastatic HER2-low–expressing breast cancer | 54 | ORR: 37.0%; median duration of Response: 10.4 months | Total: 63.0%; decreases in neutrophil; platelet; WBC counts; anemia; hypokalemia; AST increase; decreased appetite; diarrhea |
| Phase 3 trial [56] | Trastuzumab deruxtecan vs. the physician’s choice of chemotherapy | Second- or later-line | HER2-low metastatic breast cancer | 557 | PFS: 10.1 months vs. 5.4 months; OS: 23.9 months vs. 17.5 months | Total: 52.6% vs. 67.4%; Neutropenia: 13.7% vs. 40.7%; anemia: 8.1% vs. 4.7%; Nausea: 4.6% vs. 0%; fatigue: 7.5% vs. 4.7% |
| Phase 3 trial [57,58] | Olaparib vs. single-agent chemotherapy | Third-line | HER2-negative mBC with gBRCAm | 302 | ORR: 59.9% vs. 28.8%; OS: 19.3 vs. 17.1 months; PFS: 7.0 vs. 4.2 months | Anemia: 36.6% vs. 50.5% |
| Phase 2 trial [59] | Olaparib | First-line | TNBC | 32 | ORR: 56.3% ORR: (BRCA mutations vs. not BRCA mutations): 88.9% vs. 11.1% | Fatigue: 3% |
| Phase 2 trial [60] | Olaparib | NR | Advanced ovarian carcinoma or TNBC | 91 | NR | Nausea, fatigue, vomiting, decreased appetite |
| Phase 3 trial [61] | Veliparib + carboplatin + paclitaxel vs. carboplatin + paclitaxe | Third- or later-line | Advanced HER2-negative BC with gBRCAm | 513 | ORR: 75.8% vs. 74.1%; OS: 33.5 vs. 28.2 months; PFS: 14.5 vs. 12.6 months | Neutropenia: 81% vs. 84%; anemia: 42% vs. 40%; thrombocytopenia: 40% vs. 28% |
| Phase 3 trial [62] | Talazoparib vs. single-agent chemotherapy | Second- or later-line | Advanced BC with gBRCAm | 431 | ORR: 62.6% vs. 27.2%; PFS: 8.6 vs. 5.6 months | Primary anemia: 55% vs. 38%; nonhematologic: 32% vs. 38% |
| Phase 2 trial [63] | Talazoparib | Third-line | Advanced BC with gBRCA m | 84 | ORR (TNBC): 26% | Anemia; thrombocytopenia; neutropenia |
| Phase | Intervention | Line of Therapy | Patients | N | Efficacy | Safety (AE ≥ Grade 3) |
|---|---|---|---|---|---|---|
| Phase 3 trial [91] | Pembrolizumab vs. chemotherapy | Second- or third-line | mTNBC | 622 | (1) CPS ≥ 10: ORR: 18% vs. 9%; OS: 12.7 vs. 11.6 months (2) CPS ≥1: ORR: 12% vs. 9%; OS: 10.7 vs. 10.2 months | Anemia: 1% vs. 3%; decreased white blood cells: <1% vs. 5%; decreased neutrophil count: <1% vs. 10%; neutropenia: 0% vs. 13%; |
| Phase 3 trial [92] | Pembrolizumab + chemotherapy vs. chemotherapy | First-line | mTNBC | 847 | (1) CPS ≥ 10: PFS: 9.7 vs. 5.6 months; (2) CPS ≥ 1: PFS:7.6 vs. 5.6 months | Total: 68% vs. 67% immune-mediated AE: 5% vs. 0% |
| Phase 2 trial [87] | Pembrolizumab | Second- or later-line (Cohort A) | mTNBC | 170 | (1) Total: ORR: 5.3%; DCR: 7.6%; OS: 9.0 months; (2) PD-L1+: ORR: 5.7%; DCR: 9.5% | Diarrhea 1.8%; increased alanine aminotransferase: 1.2%; immune-mediated AE: type 1 diabetes mellitus; pneumonitis; |
| Phase 2 trial [93] | Pembrolizumab | First-line (Cohort B) | mTNBC | 84 | ORR: 21.4%; DCR: 23.8%; OS: 18.0 months; PFS: 2.1 months | Total: 9.5%; immune-mediated AE: rash |
| Phase 3 trial [94] | Atezolizumab + nab-paclitaxel vs. nab-paclitaxel | First-line | mTNBC | 902 | (1) Total: OS: 21.0 vs. 18.7 months; (2) PD-L1+: OS: 25.0 vs. 18.0 months | Neutropenia: 8% vs. 8%; peripheral neuropathy: 6% vs. 3%; decreased neutrophil count: 5% vs. 4%; fatigue: 4% vs. 3% |
| Phase 3 trial [95] | Atezolizumab + paclitaxel vs. paclitaxel | First-line | PD-L1-positve mTNBC | 651 | ORR: 63% vs. 55%; OS: 22.1 vs. 28.3 months; PFS: 6.0 vs. 5.7 months | Total: 53% vs. 46% |
| Phase 2 trial [96] | Pembrolizumab + radiotherapy | NR | mTNBC | 17 | ORR: 17.6%; PFS: 2.6 months; OS: 8.25 months | Fatigue; lymphopenia; infection |
| Phase 2 trial [97] | Niraparib + pembrolizumab | NR | mTNBC | 55 | ORR: 21%; PD-L1+: 32%; PFS: 2.3 months | Anemia: 18%; thrombocytopenia: 15%; fatigue: 7% |
| Phase 2 trial [98] | Pembrolizumab plus enobosarm | NR | AR-positive mTNBC | 16 | ORR: 13%; OS: 25.5 months; PFS: 2.6 months | Musculoskeletal ache: 6%; dry skin: 6%; diarrhea: 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Bao, C.; Huang, L.; Wei, J.-F. Current Therapeutic Strategies for Metastatic Triple-Negative Breast Cancer: From Pharmacists’ Perspective. J. Clin. Med. 2022, 11, 6021. https://doi.org/10.3390/jcm11206021
Li S, Bao C, Huang L, Wei J-F. Current Therapeutic Strategies for Metastatic Triple-Negative Breast Cancer: From Pharmacists’ Perspective. Journal of Clinical Medicine. 2022; 11(20):6021. https://doi.org/10.3390/jcm11206021
Chicago/Turabian StyleLi, Shuanghe, Chongyang Bao, Lingli Huang, and Ji-Fu Wei. 2022. "Current Therapeutic Strategies for Metastatic Triple-Negative Breast Cancer: From Pharmacists’ Perspective" Journal of Clinical Medicine 11, no. 20: 6021. https://doi.org/10.3390/jcm11206021




